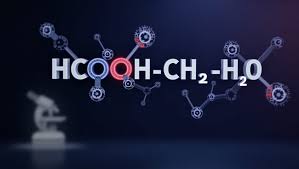
HCOOCH₃ + H₂ + H₂O: Chemistry Pathways and Industrial Significance
Introduction
Among the many fascinating compounds in organic chemistry, methyl formate (HCOOCH₃) stands out as the simplest ester and a vital intermediate in chemical industries. When combined with water (H₂O) or hydrogen (H₂), it participates in reactions that are not only central to organic synthesis but also essential to large-scale manufacturing of everyday chemicals.
The hydrolysis of HCOOCH₃ with water yields formic acid and methanol, while its hydrogenolysis with hydrogen gas produces two molecules of methanol. These seemingly simple transformations form the backbone of industrial processes ranging from solvent production to renewable fuel synthesis.
In this article, we will deeply analyze the chemistry of HCOOCH₃ + H₂ + H₂O, explore their reaction mechanisms, compare hydrolysis and hydrogenolysis, and highlight their importance in both academic research and industrial applications.
Understanding Methyl Formate (HCOOCH₃)
Structure and Composition
Methyl formate is an ester derived from formic acid (HCOOH) and methanol (CH₃OH). Its structure is represented as:
H–C(=O)–O–CH₃
- Functional group: Ester (-COO- linkage).
- Molecular weight: 60.05 g/mol.
- Appearance: Clear, colorless, volatile liquid.
- Odor: Fruity, pleasant smell often compared to rum.
Physical and Chemical Properties
- Boiling point: ~32 °C (making it highly volatile).
- Density: Around 0.97 g/cm³.
- Solubility: Partially miscible in water but readily dissolves in organic solvents.
- Reactivity: Due to its ester group, it readily undergoes nucleophilic substitution and reduction reactions.
These characteristics explain why methyl formate is both useful as a solvent and highly reactive in chemical processes involving water and hydrogen.
Hydrolysis of HCOOCH₃ with Water
Hydrolysis is one of the most common transformations esters undergo. When methyl formate reacts with water, the ester bond is broken, leading to two valuable products.
Balanced Reaction
HCOOCH₃ + H₂O ⟶ HCOOH + CH₃OH
Products:
- Formic acid (HCOOH) – simplest carboxylic acid.
- Methanol (CH₃OH) – industrial alcohol and versatile solvent.
Mechanistic Pathways
- Acid-Catalyzed Hydrolysis:
- Protonation of carbonyl oxygen increases electrophilicity of the carbonyl carbon.
- Water attacks the carbonyl carbon.
- Tetrahedral intermediate forms and collapses.
- Final step releases formic acid and methanol.
- Protonation of carbonyl oxygen increases electrophilicity of the carbonyl carbon.
- Base-Catalyzed Hydrolysis (Saponification):
- Hydroxide ion directly attacks the carbonyl carbon.
- Intermediate decomposes to give formate ion (HCOO⁻) and methanol.
- The formate ion can then be protonated to form formic acid.
- Hydroxide ion directly attacks the carbonyl carbon.
Importance of Hydrolysis
- Efficient way to manufacture formic acid, used in agriculture, leather treatment, and textiles.
- Co-production of methanol, an essential fuel and solvent.
- Showcases ester reactivity and is a standard example in organic chemistry education.
Hydrogenolysis of HCOOCH₃ with Hydrogen
Hydrogenolysis is another key transformation, where hydrogen gas reduces the ester bond, producing methanol as the only product.
Balanced Reaction
HCOOCH₃ + 2 H₂ ⟶ 2 CH₃OH
Here, both the carbonyl and ester bonds of methyl formate are reduced completely to yield two methanol molecules.
Reaction Conditions
- Catalysts: Typically copper-based (Cu/Cr, Cu/ZnO/Al₂O₃).
- Temperature: 200–300 °C.
- Pressure: High hydrogen pressure is required.
Mechanism Overview
- Hydrogen molecules adsorb and dissociate on the catalyst surface.
- Ester interacts with the active sites of the catalyst.
- Stepwise hydrogenation breaks the ester bond and reduces the carbonyl group.
- Final products desorb as methanol molecules.
Industrial Significance
- A direct pathway to methanol production.
- Methanol’s role:
- A clean-burning fuel and fuel additive.
- A precursor for formaldehyde, acetic acid, methyl tert-butyl ether (MTBE).
- A key material in plastics, resins, and adhesives.
- A clean-burning fuel and fuel additive.
- Provides an alternative to the CO + H₂ (syngas) route of methanol production.
Hydrolysis vs. Hydrogenolysis: A Comparative View
Both hydrolysis and hydrogenolysis start with methyl formate, but the conditions, catalysts, and products differ significantly.
| Feature | Hydrolysis (H₂O) | Hydrogenolysis (H₂) |
| Catalyst | Acid/Base | Metal (Cu/Cr, Cu/ZnO) |
| Reaction Type | Nucleophilic substitution | Reductive cleavage |
| Products | Formic acid + Methanol | Methanol only |
| Reversibility | Acid catalysis reversible | Irreversible |
| Industrial Use | Formic acid production | Methanol synthesis |
This comparison shows how selective use of water or hydrogen directs the pathway toward different but equally valuable products.
Industrial Applications of HCOOCH₃ Chemistry
1. Formic Acid Production
- Obtained from hydrolysis of methyl formate.
- Uses:
- Preservative in livestock feed.
- Leather tanning and textile finishing.
- Cleaning and descaling agents.
- Preservative in livestock feed.
2. Methanol Production
- Derived from hydrogenolysis.
- Uses:
- Green fuel and biodiesel component.
- Feedstock for formaldehyde, MTBE, and plastics.
- Solvent in pharmaceuticals and paints.
- Green fuel and biodiesel component.
3. Green Solvents and Blowing Agents
- Methyl formate itself is marketed as an eco-friendly solvent.
- Used as a blowing agent in polyurethane foams, replacing ozone-depleting substances.
Safety, Handling, and Environmental Considerations
- Flammability: Highly flammable liquid due to low boiling point.
- Toxicity: Inhalation can cause dizziness, nausea, and respiratory irritation.
- Storage: Keep in cool, ventilated areas away from sparks and ignition sources.
- Environmental Impact:
- Hydrolysis products are biodegradable.
- Hydrogenolysis contributes to clean methanol synthesis, aligning with green chemistry principles.
- Hydrolysis products are biodegradable.
Research and Future Outlook
Recent research is exploring:
- Advanced catalytic systems for more energy-efficient hydrogenolysis.
- Bio-based methyl formate production from renewable resources like biomass or captured CO₂.
- Integration of methyl formate reactions into carbon capture and utilization (CCU) strategies.
- Expanding its role as a sustainable solvent in eco-friendly industrial processes.
The dual role of methyl formate as both a chemical intermediate and a final product ensures that its relevance will only grow in future energy and chemical landscapes.
Conclusion
The chemistry of HCOOCH₃ + H₂ + H₂O provides a powerful example of how a single compound can lead to vastly different outcomes depending on the conditions. Hydrolysis with water produces formic acid and methanol, while hydrogenolysis with hydrogen leads to exclusive methanol formation.
These reactions are not just textbook examples of ester chemistry—they are cornerstones of industrial chemistry, enabling the large-scale manufacture of essential chemicals. Moreover, their integration into green chemistry and renewable energy frameworks highlights their importance in a sustainable future.
In short, methyl formate stands as a bridge between fundamental organic chemistry and practical industrial innovation, making the study of HCOOCH₃ + H₂ + H₂O essential for both academic and industrial chemists.